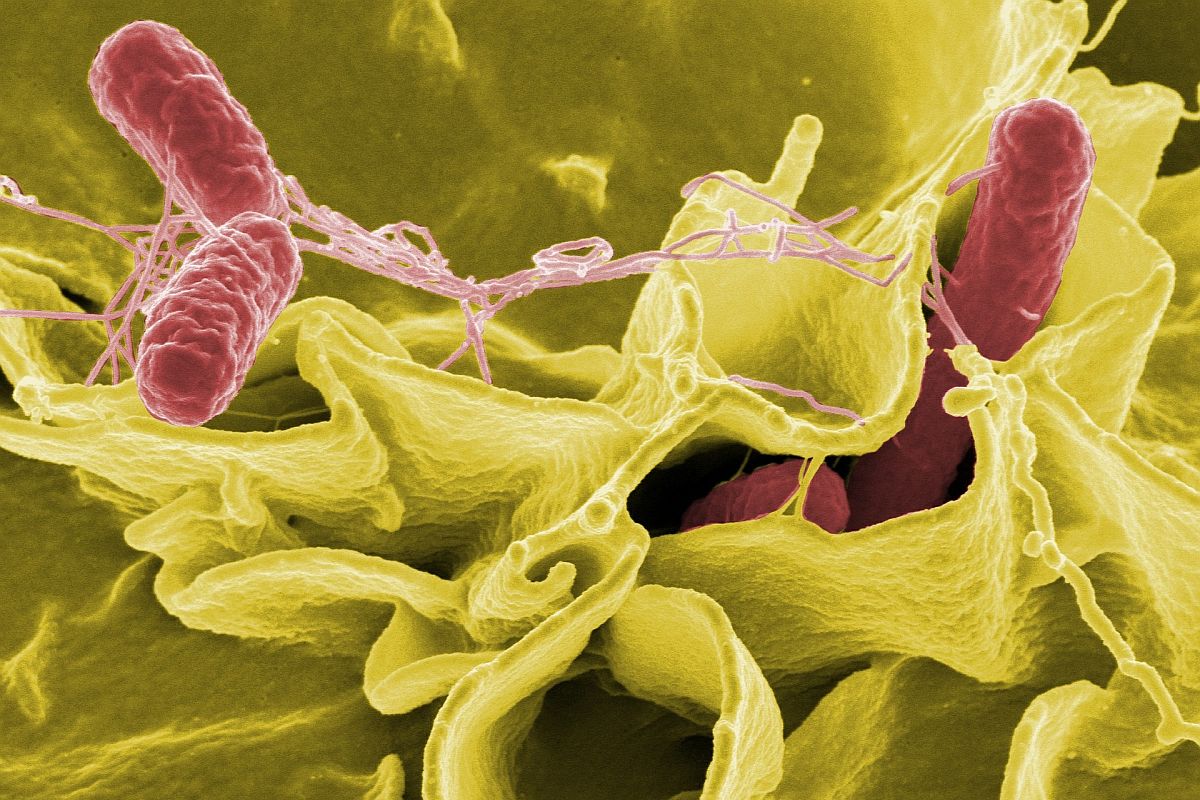
Strict cleaning regimes eliminate the risk of endoscopes transmitting bacteria like Salmonella
Cleaning comes first
In 1993 a report attributed 281 episodes of pathogen transmission in the USA to gastrointestinal endoscopy. The FDA introduced strict rules and the rate of infection fell to 1 in 1.8 million procedures in 10 years. In the next decade to 2013 there were about 3 reported cases of infection in over 30 million procedures per annum.
Every case after 2003 was linked to a breach of at least one step in the high level disinfection rules. The process is pre-cleaning and leak testing, cleaning and rinsing, disinfection and rinsing, drying and storage.
In March 2016 the Department of Health updated its guidance on the decontamination of flexible endoscopes following advice from the Advisory Committee on Dangerous Pathogens.
This guidance included best practice recommendations, design and fitting of endoscope reprocessing units, advice on the use of endoscope washer disinfectors, tests needed to prove decontamination, and testing methods.
Three of its top tips are:
Compatibility.
Endoscopes must be compatible with decontamination processes including the endoscope washer-disinfectors.
Manufacturer's instructions.
All equipment must be operated, cleaned in accordance with the manufacturer’s instructions and local procedures
Track and trace.
Auto-identification and associated data capture should be used to track all endoscopes, reusable accessories, decontamination, and use with patients.
Requirements
For the decontamination process to succeed every time endoscopy departments need the right cleaning materials for each type of endoscope and washer disinfector. They depend on specialist suppliers to work with the equipment manufacturers to ensure everything is suitable.
Identification and tracking are essential to control the process.
Solution
The operations manager of the company came to us as Codeway was able to supply a complete solution at a reasonable cost.
Labels
They had been using preprinted labels but these were unsuitable for tracking batches, dates and individual items. We worked with the operations manager to specify the labels the company needed to identify its range of products. These were a multi-part, 'piggy back', design to meet its customers' needs for traceability.
Label printing
Codeway supplied Bartender software for the company to design and modify products. This drives a Zebra printer with an internal rewind to produce rolls of labels for each batch of product. To ensure continuity of production Codeway provided a full, on-site maintenance contract.
Stock tracking
The next step in the project company is stock tracking. The company will scan batches of product into its warehouse, pick and pack orders, and scan despatches out to customers.
Kitting
Infection control managers are promoting the use of kits for medical and surgical procedures. They prevent use of the wrong item. The company is planning use its stock tracking system to provide a kitting service for its customers.